...on the rationalisation, design, optimisation and synthesis of novel phosphonium derived anti-cancer drugs
 |
Establishing Anti-Cancer Phosphonium Salt Structure-activity RelationshipsPart 3by Phil |
Establishing Structure and Quantity-Activity Relationships
3.1 Potency as a function of Charge
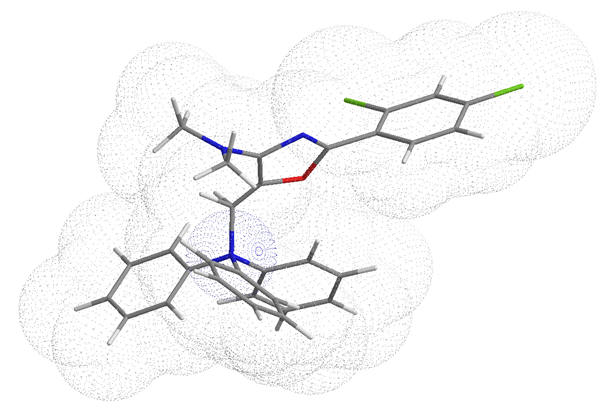
According to Equation 6, the hydration enthalpy varies as a square law of charge whilst only linearly with radius. In order for the desolvation energy of a dication to remain favourable, the lipophilicity would have to increase proportionally higher than charge. This explains why the dimeric TPP (see Table 2) is significantly less active than the corresponding mono phosphonium and also less potent than similar structural homologues of similar lipophilicity.[64]
Table 2 | Comparison of a dicationic to monocation TPP dimer pair
 |
 |
|
| CLogP | 9.49 | 4.62 |
| IC50 | 2.4 | 0.8 |
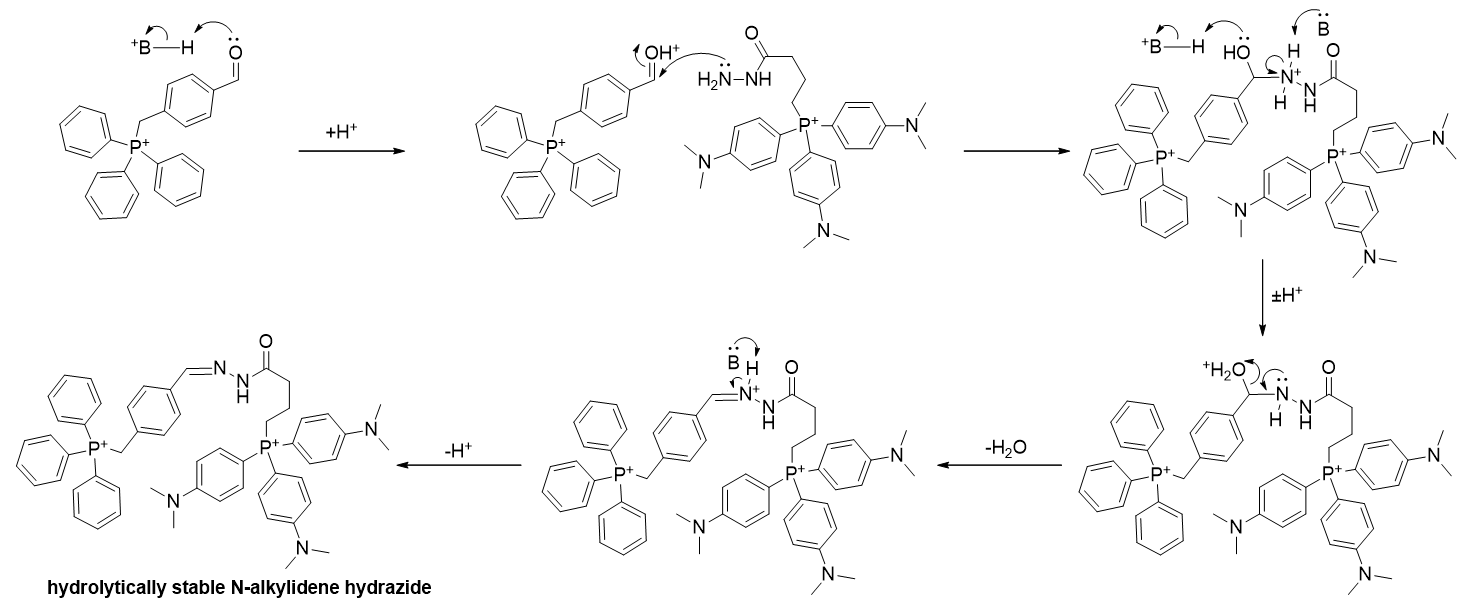
In related dicationic phosphoniums, to overcome the increased solvation energy a successfully applied strategy was to increase lipophilic surface. [56] Another strategy that circumvents the uptake issues associated with polycations but exploits the increase accumulation is self-assembly of polycationic TPPs from monocationic precursors. An example of a self-assembled dication has been reported to accumulate 10x greater than the sum of the individual monocations or that of preincubated monomers, [57] which suggests sub-mitochondrial assembly takes place. Mechanistically, self-assembly is a result of condensation of a hydrazine derivatised phosphonium and an aldehyde derivatised phosphonium to yield a hydrolytically stable [58] alkylidene-alkylhydrazide.
Scheme 1 | Self-assembly of monocationic TPPs affording a stable hydrazide
There have been only few reports on the activity of dicationic phosphonium. However one pair of reported bisphosphoniums were found that in accord with the Nernst equation for dications accumulation varied ten-fold per 30mV. [59]
3.2 Potency as a function of lipophilicity
It is generally assumed that the efficacy of an agent is a function of it concentration at its site of action. TPPs that achieve high matrix concentrations before systemic elimination are likely to be more potent. Potency may therefore be a function of accumulation, which is a function of the Born energy. According to Equation 6, hydration is inversely related to ionic radii and ions of large radii are more lipophilic.
The LogP is a measure of lipophilicity determined as the equilibrium distribution between water and 1-octanol (see Equation 7). LogP can be estimated computationally and these calculated logP values (CLogPs) are frequently used to establish quantitative structure activity relationships and for structure-property correlation.
Equation 7 | Definition of logP
$$\log P = {\log _{10}}{P_x} = {\log _{10}}\left( {\frac{{{{[x]}_{{\text{n - octanol}}}}}}{{{{[x]}_{{\text{water}}}}}}} \right)$$
Many tools are available to estimate properties in silico, but molInspiration (miLogP) has been employed successfully elsewhere to investigate QSAR models of anticancer drugs [61] and so is used here. Ions of salts generally dissociate from one another upon dissolution and thus the counter-anion should not contribute to lipophilicity of the active agent. [60]
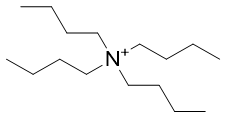
Series of tetralkylated cations
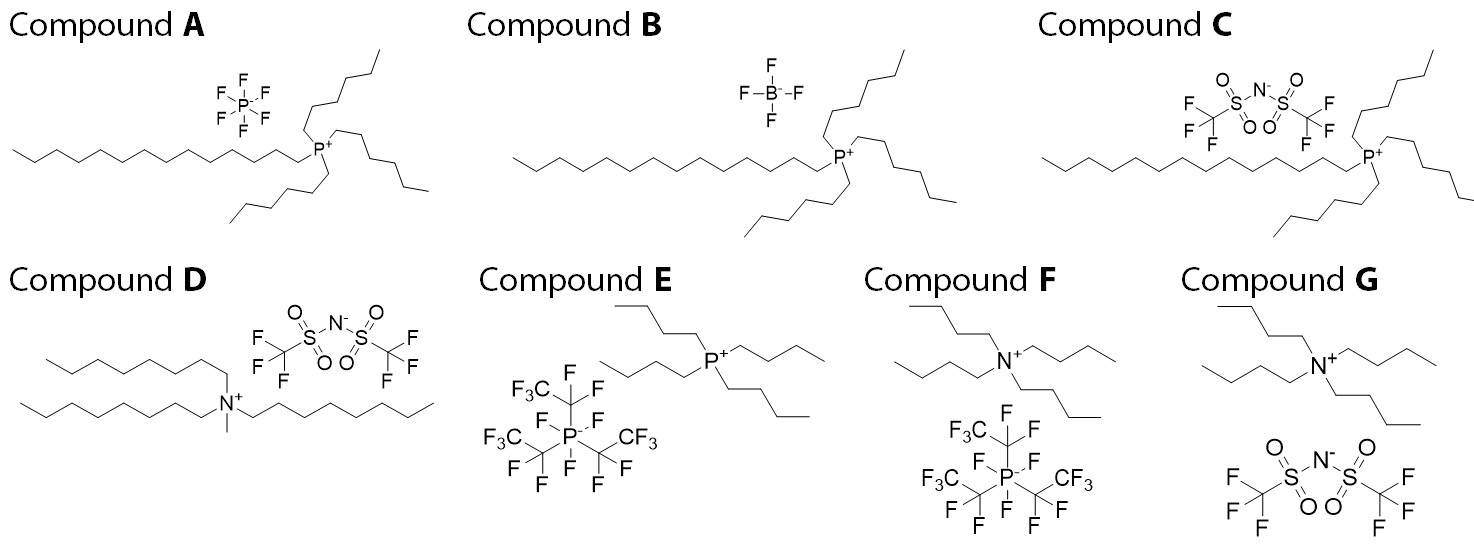
Estimation of CLogP were performed using the computational methods established by Tetko et. al. on the Ionic liquids (IL’s, see Figure 10) reported by Kumar et. al. [63] some of which exhibit modest anti-cancer effects.
Figure 10 | Ionic Liquids investigated for anti-tumour activity (Kumar et. al.)
 |
 |
 |
 |
|
| CLogP | 9.1 | 6.52 | 5.62 | 3.54 |
| 1 / GE50 | 0.025 | 0.039 | 0.046 | 0.173 |
Figure 11 | Phosphonium and Ammonium Cations in some commercial Ionic Liquid
Table 3 | Anti-tumour activity (1/GI50%) of select Ionic Liquids (Kumar et. al.)
| Compound: | A | B | C | D | E | F | G |
| ↓ Cell Line | CLogP → | 9.1 | 9.1 | 9.1 | 5.62 | 6.52 | 3.54 | 3.54 |
| HL-60 (TB) | 40.0 | 25.6 | 21.7 | 5.8 | 0.4 | Inactive | Inactive |
| LOX-IMVI | 27.0 | 20. | 17.2 | 5.2 | 0.2 | ||
| SK-MEL-5 | 19.2 | 13.5 | 12.7 | 5.7 | 0.4 | ||
| PC-3 | 28.6 | 22.7 | 22.2 | 3.6 | 0.3 |
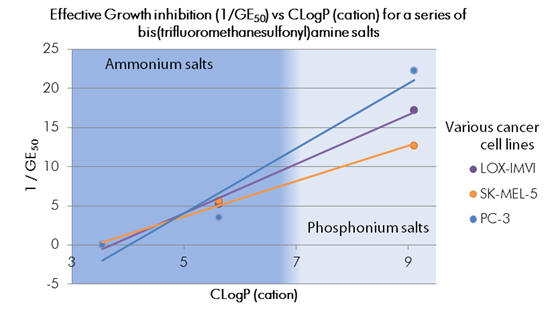
For a subseries of compounds with common counter anions (C, D & G, Figure 10), potencies increase with logP (see Graph 6). Evidently, not only phosphonium salts exhibit anticancer effects, the key feature being presumably the cation charge.
Graph 6 | Antitumour activity of IL’s as a function of cation CLogP for salts of common anion
Compounds of the cations 1, 2 and 3 displayed antitumour activity whilst compound 4 was inactive (see Table 3). Cation 4 being the least lipophilic, according to the Born model would be least capable of membrane penetration. There may be a lipophilicity ‘cut-off’ point below which agents are too strongly solvated for adsorption. On-going from cation 4 to the more lipophilic cation 3 the anti-proliferative effects become evident, albeit modestly. Following the trend cation 1 is more potent than the less lipophilic cation 2. Over this small series Potency increases with lipophilicity, although simple alkyl derivatives are known to be toxic.
Extended series of complexly substituted TPPs
Using similar methods (Tetko, loc cit) more phosphonium salts were analysed (Neamati et. al., see Appendix 1 Additional Structures and Data). [64]
Figure 12 | A representative sample of anti-cancer TPPs (Neamati et. al.)
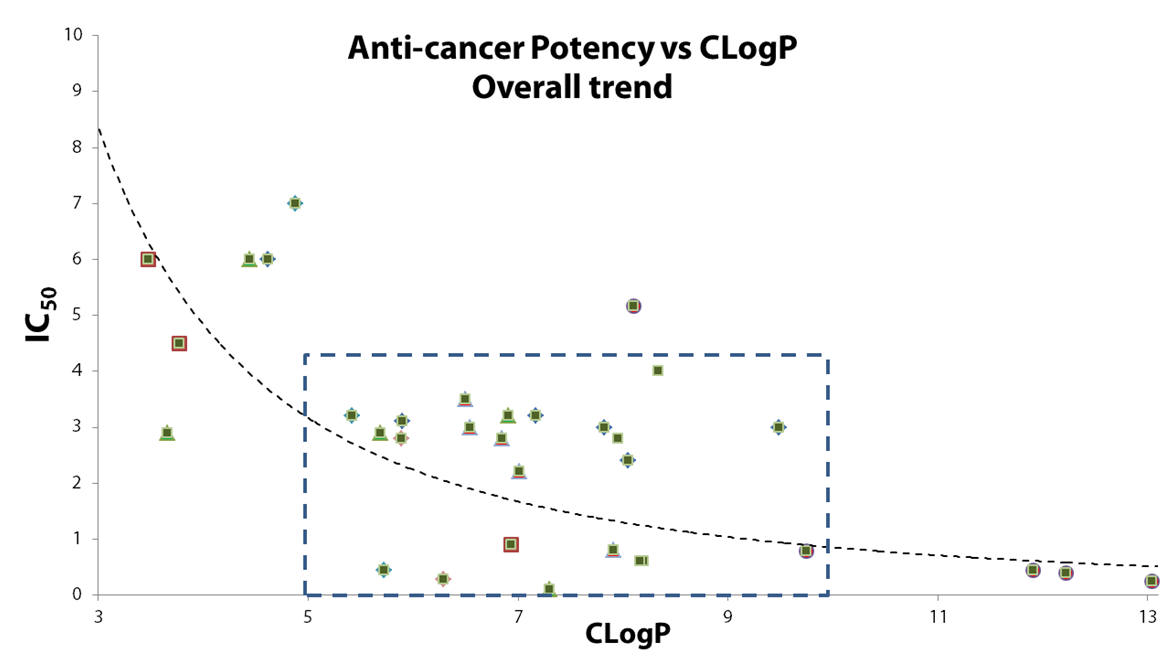
Analysis of the complete dataset yields information of statistical significance, Graph 7 shows:
- It is rare for potent compounds to have a low CLogPs
- It is common for compounds which have CLogP > 5 to have IC50 < 3
- The most potent aliphatic analogues have intermediate lipophilicity, 5.5 > CLogPs > 8.5
Graph 7 | IC50 as a function of CLogP of all TPPs (see appendix for all structures)
It’s unlikely to possible to directly compare structurally diverse compounds (see Figure 12) because effects of uncommon modes of molecular interaction would complicate analysis (H-bond donor and acceptor numbers, molecular mass range and counter-ion), but for series of related structures reliable analysis can be achieved. To investigate these specific trends, sub-series were grouped as per Figure 13 and the analysis repeated (Graph 8).
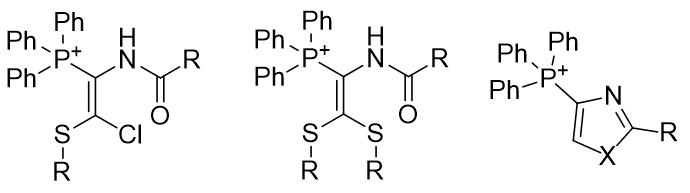
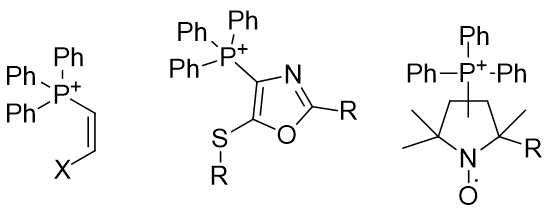
Figure 13 | Groupings of phosphonium salts into structural series
Graph 8 | IC50 as a function of CLogP – trends for individual series
Across the subseries’ there are general trends for potencies to increase with lipophilicity. At lower lipophilicities potencies drop significantly, whereas at the high lipophilicity increases had little effect (Graph 8). This suggests a potency-maximum for each series, beyond which structural modification cannot raise potency. The members of each family are below.
1. Thiochlorovinyl amides
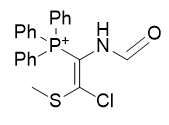 |
 |
 |
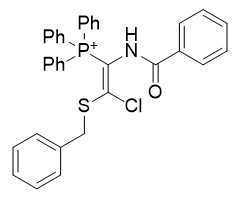 |
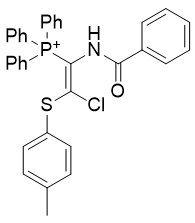 |
|
| Compound | TP-812 | TP-752 | TP-728 | TP-794 | TP-749 |
| CLogP | 3.66 | 4.44 | 5.69 | 6.91 | 7.3 |
Figure 14 | A series of thiochlorovinylamides
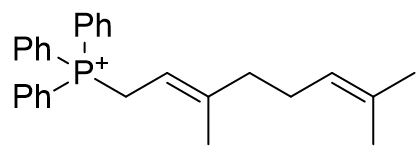
2. Olefins
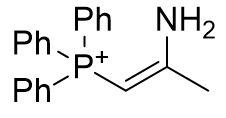 |
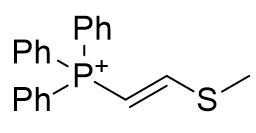 |
 |
|
| Compound | TP-764 | TP-825 | TP-740 |
| CLogP | 3.48 | 3.77 | 6.93 |
Figure 15 | A series of olefins
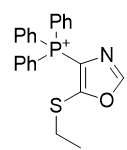
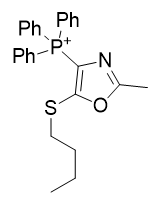
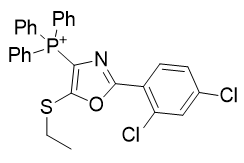
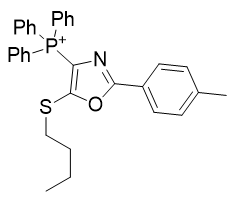
3. Thio-oxazoles
 |
 |
 |
 |
 |
 |
|
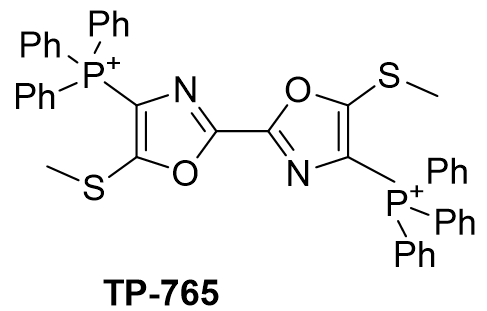
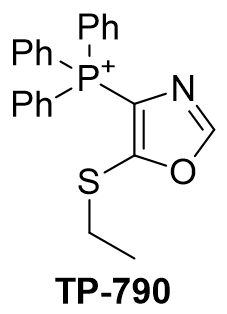
| Compound | TP-790 | TP-744 | TP-737 | TP-736 | TP-726 | TP-765 |
| CLogP | 4.62 | 5.9 | 7.17 | 7.82 | 8.05 | 9.49 |
| IC50 | 6 | 3.1 | 3.2 | 3 | 2.4 | 2.8 |
Figure 16 | A series of thio-oxazoles
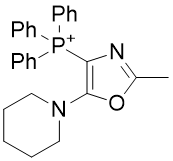
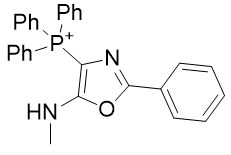
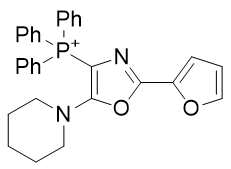
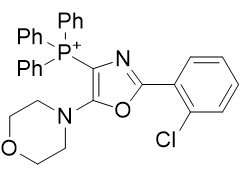
4. Oxazole amines
 |
 |
 |
 |
|
| Compound | TP-821 | TP-768 | TP-769 | TP-801 |
| CLogP | 4.88 | 5.42 | 5.72 | 6.14 |
Figure 17 | A series of Oxazole amines
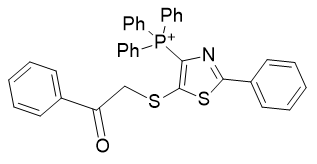
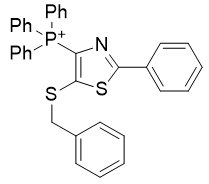
5. thio-thiazoles
 |
 |
 |
 |
|
| Compound | TP-822 | TP-760 | TP-824 | TP-781 |
| CLogP | 7.95 | 8.16 | 8.2 | 8.34 |
Figure 18 | A series of thio-thiazoles
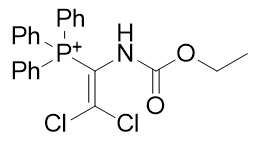
6. dichlorovinyl carbamates
 |
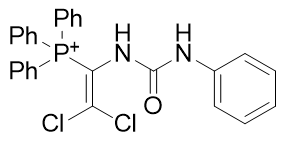 |
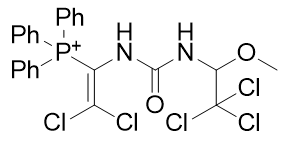 |
|
| Compound | TP-785 | TP-738 | TP-835 |
| CLogP | 4.81 | 5.71 | 5.82 |
Figure 19 | A series of dichlorovinyl carbamates
Pairs of TPP analogues
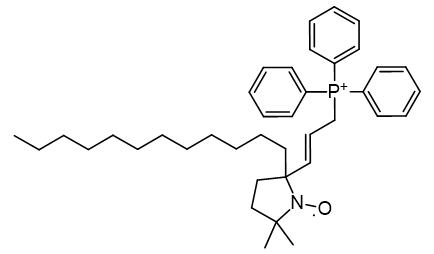
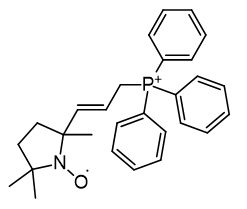
Of a series TEMPO phosphoniums investigated for anti-cancer efficacy, [65] only more lipophilic members displayed efficacy. Comparison of HO-3814 to HO-4223 clearly illustrates that incorporation of the C11 linker is to enhance lipophilicity rather than adjust the relative molecular distances.
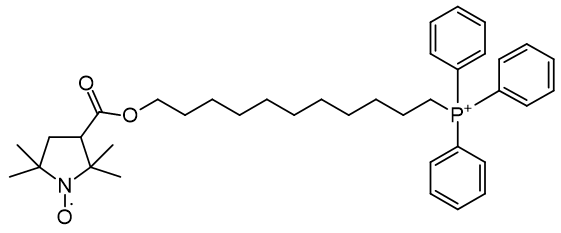 |
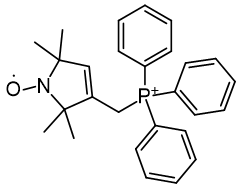 |
|
| Compound | Mito-CP | HO-435 |
| Cell viability | 60% | 100% |
| CLogP | 5.9 | 2.57 |
 |
 |
|
| Compound | HO-3814 | HO-4223 |
| Cell viability | 50% | 100% |
| CLogP | 7.67 | 2.29 |
Figure 20 | Anticancer MitoQ-CP homologues
Ring bioisosteric substitution and derivatisation
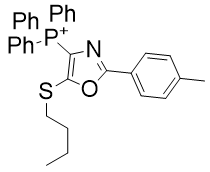
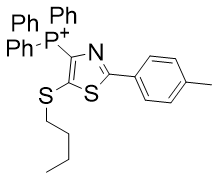
Despite the subtle differences between these diazoles, they are sufficiently different that they cannot analysed together. Across the set azoles below there is no trend with logP (see Graph 9).
 |
 |
|
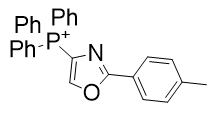
| Compound | TP-726 | TP-824 |
| CLogP | 8.05 | 8.2 |
| IC50 | 2.4 | 0.6 |
 |
 |
|
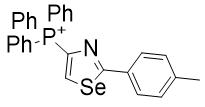
| Compound | TP-772 | TP-731 |
| CLogP | 5.89 | 6.29 |
| IC50 | 2.8 | 0.28 |
Figure 21 | A set of diazoles
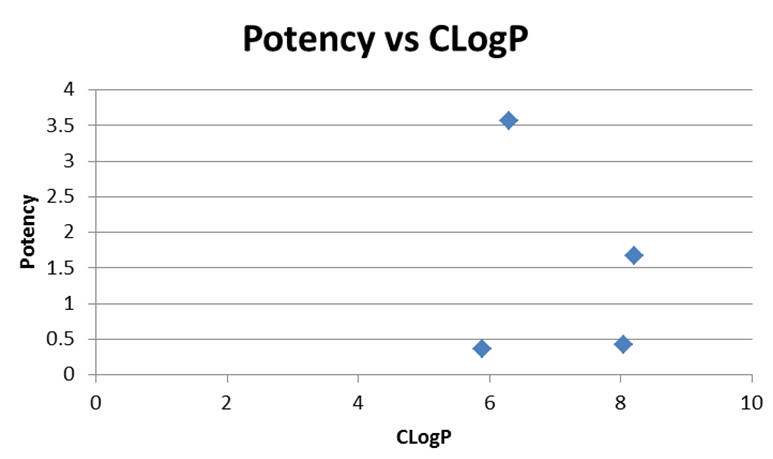
Graph 9 | 5-substituted-diazoles
Trends appear when pairs of molecules are analysed independently. Substitution of the ring heteroatom with a heavier congener results in a gain in potency larger than the change in lipophilicity would suggest, for example:
- Substitution of oxygen in TPP-726 with sulphur to give TP-824 raises CLogP by 2% but raises potency by 300%
- Substitution of oxygen in TP-772 with selenium to give TP-731 raises CLogP by 7% but raises potency by 1000%
Contrastingly, derivatisation of TP-772 with the butylthio group to give TPP-726 raises the CLogP by 36% but potency only by 8%. Homologation increases potency as expected (see Figure 22) but the introduction of unsaturation results in significant gains in potency (see Figure 23).
 |
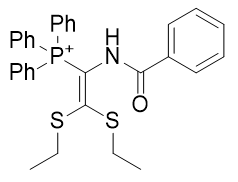 |
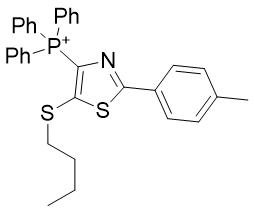 |
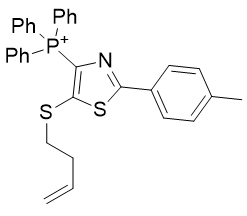 |
|
| Compound | TP-791 | TP-734 | TP-824 | TP-760 |
| CLogP | 5.23 | 5.99 | 8.2 | 8.16 |
| IC50 | 5 | 0.1 | 1.5 | 0.18 |
Figure 22 | Alkyl group Homologation (left) and Figure 23 | Introduction of unsaturation (right)
3.3 Summary
In summary these analyses point towards lipophilicity as a principle feature for drug optimisation. No light has been shed on the pharmacophore but several important variables which directly determine TPP activity have been identified. Based on this review some guidelines have emerged (see Table 4)
Phosphonium Design and Optimisation Guidelines
- Cancer selectivity is poor for polycations
- Monocations accumulate with excellent selectivity
- Large lipophilic ions are capable of direct membrane traversal
- Potency tends to increase with lipophilicity
- The very most potent compounds typically have 7.5 > CLogP > 8.5
- Generally active compounds have intermediate lipophilicity, 5.5 > CLogP > 9
- For each series of compounds, potency tends towards nil at low CLogP and tend towards a plateau at high CLogP
- Heavier heteroatoms in cycles yield more potent analogues
- Bioisosteric substitution provides larger gains than homologation
- Unsaturation enhances potency
- Homologation enhances potency