...on the rationalisation, design, optimisation and synthesis of novel phosphonium derived anti-cancer drugs
 |
Establishing Anti-Cancer Phosphonium Salt Structure-activity RelationshipsPart 1by Phil |
Preface
Cancer affects nearly 300,000 people per year in the UK.[1] In the UK around 1 million people have newly diagnosed cancer.
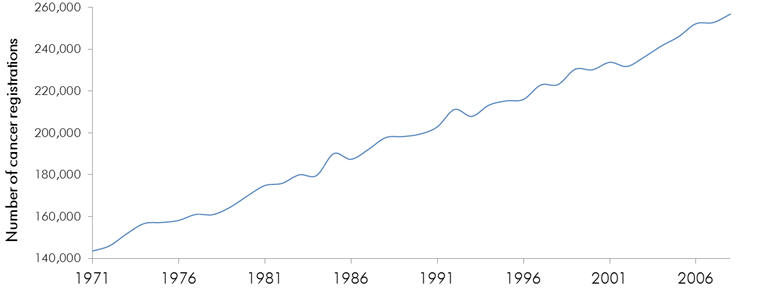
Figure 1 | Number of newly diagnosed cancers
in England (1971-2008)
Source: Registrations of cancer diagnosed
in 2008, England, Office for National Statistics, pp19
Potent and toxic drugs are used to treat cancer. There can be a fundamental difficulty in selectively targeting tumour tissues and so side-effects, often serious are common-place. Some tumours exhibit drug resistant phenotypes and others develop resistance [2] and this can render whole families of drugs ineffective. There is a pressing need for new non-toxic medications independent of cross resistance to existing anti-neoplastic drugs.[3]
Introduction
1.1 What are Triphenylphosphonium salts?
Triphenylphosphonium (TPP) salts are a class of well-known, structurally simple and non-toxic [4] chemicals containing the cationic phosphonium group, see Figure 2.


Figure 2 | Representative phosphonium anticancer agents
These are employed extensively in synthetic chemistry, the most especially famed application being the Wittig reaction.[5] Over the recent decades many TPPs have emerged to exhibit potent and selective anticancer properties.[6] In animal experiments within minutes of intravenous TPP administration, accumulation to the site of action occurs. Within one hour concentrations had reached a level for anti-cancer effects to be observed.[4] TTPs are an exciting new family of drugs, and many of which are in preclinical study – candidates are sought to be taken forwards to clinical trials.[64]
TPPs induce cell death effectively, but by an unknown mechanism
Our understanding of the molecular mode of action is sparse. Fluorescence studies [7] and 31P-NMR has demonstrated accumulation to the mitochondria,[8] and it is here the agents derive their efficacy. TPPs uptake increases with time and suggesting an increase in mitochondrial permeability may be an indicator of drug-effect.[13,59] Currently no drug-receptor complexes have been identified and the specific interactions that underpin activity are unknown.
TPPs are cationic species and as such are up-taken into the mitochondria
Since it was demonstrated that organic cations accumulate in the mitochondria [40] there has been active research into the field. Mitochondrial dysfunction is central to many disease conditions including cancer and targeting the mitochondria opens up novel drug targets to medicine.[9] The mode of delivery is a result of cationic attraction for the mitochondria by a process of electrophoretic uptake. [10] This occurs by virtue of the negative membrane potential (Δψm) [11] established across the inner mitochondrial membrane. [12] Delivery is not specific and so accumulation occurs in healthy mitochondria too (albeit to a lesser extent) but here TPPS do not appear to have toxic effects. [13]
TPPs as anticancer drugs
Phosphonium salts seem to possess a winning combination of attributes when it comes to new drug therapies: cheap, accessible, non-toxic, selective and potent. This is a literature review on anticancer TPPs and it incorporates a review of modern reports. It is an objective to investigate structure/property activity relationships and to investigate application of the Nernst-Born model to TPP action and selectivity. The study is limited to a range of anti-cancer phosphonium salts and the scope is confined to the discussion of the Nernst-Born model with attempts to correlate properties to physiological activity. The factors affecting uptake and accumulation and the factors which determine them will be identified.
1.2 Mitochondria: Structure, Function and Exploitation
Mitochondria are organelles with double-membranes which comprise two aqueous sub-compartments, an internal matrix and the inter-membrane space. [14] They are central to the proper cell functioning and their principle function is the production of ATP. [15]
Figure 3 | Structure of mitochondria
The membranes are comprised primarily of phospholipids and other fatty molecules, and as such the membranes are impermeable to hydrophilic species. The inner membrane protrudes in convoluted lamellae called cristae [16] which maximises the area exposed [17] to the apparatus that facilitate aerobic respiration, [18,19] There are countless possible sub-mitochondrial targets at which TPPs act, [20] until recently only few of which have been exploited. [21]
The Membrane Potential
At the expense of catabolic energy, water molecules in the matrix are ionised, and the proton transported out. [22] An asymmetric membrane distribution of protons and other ions results [23] but also the establishment of an electrochemical potential gradient (below). [24]
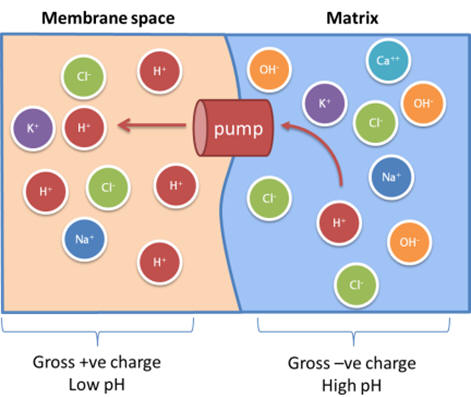
Figure 4 | Electron transport mechanisms maintain an asymmetric ion distribution
The matrix has a negative charge [11] and the magnitude of the potential energy established is quantified as the membrane potential (Δψm). Δψm of various compartments have been measured, see Table 1. [25]
Table 1 | Variation of potential in different physiological compartments
| Compartment | Electrochemical potential |
| Plasma | 0mV |
| Cytosol | -30mV |
| Matrix (healthy cell) | -170mV to -200mV |
| Matrix (cancer cell) | -200mV to -270mV |
In the normal mitochondria, Δψm is between 140 and 170mV lower than cytosol, [26] see Figure 5. Carcinomal mitochondria exhibit characteristic abnormalities; [27] Δψm can be exaggerated up to 70mV. [28,29]
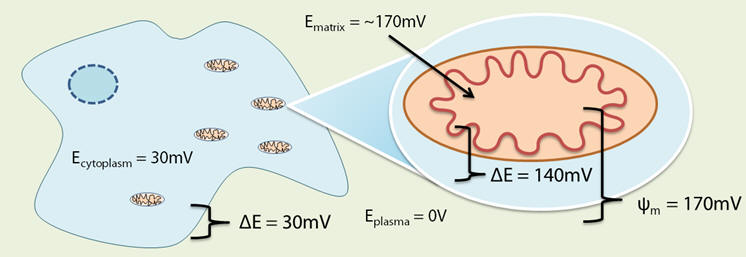
Figure 5 | Relative compartmental potentials showing some detail of a mitochondria
In vivo the transmembrane potential is harnessed to attract protons to drive ATP synthesis. If Δψm drops too close to zero mitochondrial ATP production tapers.[13] Prolonged reductions in Δψm activate the membrane permeability transition [30] which causes unrestricted and complete depolarisation. [31] The mitochondria produce an abundance of reactive oxygen species (ROS, signs of stress),[32] as they attempt to restore a stable potential. [33] ROS damages key apparatus and once this has happened the depolarisation is irreversible [34] and the mitochondrial initiate cell-wide apoptosis. [35]